Meeting Reuse Requirements
About the author: Jens Scheideler is advanced oxidation processes manager for Xylem Inc. Scheideler can be reached at [email protected] or +49 5221 930 721.
The application of ultraviolet (UV) advanced oxidation processes (AOPs) in direct and indirect potable reuse treatment trains is a commonly accepted technology to provide a barrier against pathogens and micropollutants that are not completely removed by physical treatment technologies.
Typically, these systems are a combination of high-intensity UV light and hydrogen peroxide, resulting in photolysis of the DNA of pathogens, photosensitive chemicals and the in-situ creation of hydroxyl (OH) radicals degrading organic constituents like 1,4-dioxane.
The combination of UV light with hypochlorite has received some attention in the academic world in recent years, and the principles of UV-based AOPs using hypochlorite to substitute hydrogen peroxide for the creation of OH radicals are well understood. The first papers about this process published a decade ago have not been implemented in full scale and papers describing its efficiency and economics for reuse applications are not yet available.
Compared to UV + H2O2 AOP the efficiency of the combination of UV with hypochlorite is dependent on the pH of the water as hypochlorite dissociates into hypochlorous acid and hypochlorite.
Both species will form OH radicals when irradiated with UV light, but hypochlorite is a strong scavenger of OH radicals and therefore the efficiency of the process decreases at higher pH levels.
This article will describe the advantages of using UV-based AOP with chlorine based on the first full-scale pilot study of this application.
Pilot Study
The objective of the pilot study was to evaluate the most economic AOP for the expansion of a 12-million-gal-per-day (mgd) advanced water purification facility, the Terminal Island Water Reclamation Plant in Los Angeles. LA Sanitation (LASAN) conducted both bench and pilot-scale studies on commercially feasible AOP technologies to meet California’s groundwater recharge regulations, which require a 0.5-log reduction of 1,4-dioxane (as an AOP surrogate) and less than 10 ng/L of N-Nitrosodimethylamine (NDMA) in the effluent. The following AOPs were tested using Xylem’s Wedeco MiPRO AOP Pilot Container: UV LP + H2O2; UV LP + NaOCl; UV MP + H2O2; UV MP + NaOCl; and ozone + H2O2.
All technologies successfully met the requirement of 0.5-log reduction of 1,4-dioxane. However, due to the need to reduce NDMA levels below 10 ng/L, it was determined that UV-based AOPs are more effective ozone-based AOPs in this water matrix. Furthermore, the UV-based AOPs provide the potential for greater disinfection credit with pathogens such as Cryptosporidium and other viruses. For the UV-based AOP pilot-scale tests, two types of UV reactors were evaluated using low-pressure, high-intensity lamps and medium-pressure lamps. Hydrogen peroxide and sodium hypochlorite (to generate free chlorine residual) were separately added to influent (i.e., reverse osmosis [RO] permeate) in the UV reactors via a peristaltic pump and static mixer. Along with UV transmittance, UV intensity and flow rate, the hydrogen peroxide and free chlorine concentrations were monitored in the process.
Results & Economics
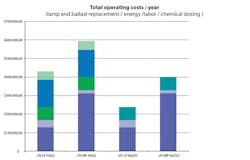
Figure 1 shows the comparison between low-pressure UV with hydrogen peroxide and hypochlorite, and medium-pressure UV with hypochlorite. The log reduction of 1,4-dioxane with low-pressure UV was practically independent of the oxidizing agent, while the performance of the medium-pressure UV reactor required three to four times more energy than low-pressure UV.
When applying hydrogen peroxide, there is a significant cost for both the addition of chemical prior to reaction and quenching of the residual chemical after reaction using hypochlorite. Because only about 10% of the hydrogen peroxide is consumed in this application, the residual typically must be disposed of prior to groundwater recharge or other potable uses. The costs for medium-pressure UV are higher than low-pressure UV due to the high energy consumption. Based on the costs for the 12-mgd plant at Terminal Island, the use of sodium hypochlorite will save LASAN approximately $80,000 per year, or $1.6 million over a typical 20-year lifecycle cost evaluation. Additionally, the Terminal Island plant already has sodium hypochlorite on site as it is a common chemical used in water and wastewater treatment.
The outcome of this study led the city of Los Angeles to implement the first UV + hypochlorite AOP system for potable reuse using two Wedeco K-MiPRO low-pressure UV reactors to treat 12 mgd of water for indirect potable reuse.
Due to its acidic pH, RO permeate provides optimal conditions for the UV + hypochlorite AOP, resulting in the creation of a sufficient OH radical yield for the degradation of 1,4-dioxane by more than 0.5 log. Compared to the classic UV + H2O2 process, the performance is similar but disposing of residual chemical is not necessary and therefore the costs associated with additional chemical dosing are not incurred. For applications in which a residual chlorine level is required and the pH of the water is less than 7, the combination of UV light and hypochlorite is an attractive concept to provide high levels of disinfection and micropollutant oxidation.