How To: Sampling & Analyzing Equipment: How to Apply & Maintain pH Sensors
About the author: Jay Mershon is senior business manager for liquid analytical products for the eastern U.S. for Endress+Hauser. Mershon can be reached at [email protected] or 610.906.1502.
This article provides a basic understanding of the application, theory, installation and maintenance requirements for pH sensors. The goal of the article is to provide the basic knowledge and guidance needed to implement a successful process pH measurement system.
pH Measurements
A pH sensor measures the hydrogen ion concentration in dilute ionic solutions. The pH measurement provides an important, real-time process variable for the monitoring and control of process pH. Reliable pH control can have a positive impact on product quality and operational costs. A dependable pH measurement also is critical in processes such as water and wastewater treatment, where the final water quality can have a significant impact on health, safety and the environment.
Chemicals, oils, greases and solids can drastically affect the performance of pH sensors. Fouling, wearing or breaking the measurement electrode and clogging or poisoning the reference junction are common in harsher applications. Sensor selection, installation and proper maintenance can extend sensor life and ensure measurement dependability.
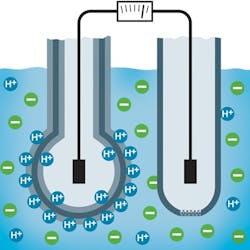
Two basic types of sensors are used. The first type uses pH-sensitive glass or enamel. These potentiometric sensors are based on a pH-sensitive membrane on which hydrogen ions accumulate and develop an electrical potential (Figure 1). An analyzer uses this electrical potential and temperature compensator input to calculate the pH value according to the Nernst equation.
The second type is an ion-sensitive field effect transistor (ISFET) pH sensor (Figure 2). The ISFET sensor uses a coated gate to isolate itself from the process. Hydrogen ions accumulate on this gate and increase the current flow through the transistor. An analyzer uses the current flow and temperature to calculate the pH value according to the Nernst equation.
How to Install pH Sensors
All pH sensors should be fully submersed at all times to avoid dehydration of the pH-sensitive membrane and hardening of process residue on the sensor. Glass pH sensors must be installed with the tip of the sensor pointing down at an angle of 15 degrees or greater.
Sensors must be removed periodically for calibration, cleaning or replacement. All pH sensors should be installed for easy access without compromising personnel safety or measurement availability. For instance, installation in a tank or process line with retraction hardware and a ball valve facilitates sensor access with no need to drain the tank or interrupt processes.
In both tank and inline installations, consider the point of reagent addition and chemical reaction time. In some cases, static mixers or agitators are employed decreasing the time required for the reagent to mix and reactions to complete prior to the measurement point, assuring a representative sample.
Measurement electrodes and liquid junction types, as well as installation hardware options, should be carefully considered for each application. Most suppliers can recommend the pH sensor and associated equipment best suited for the application. However, it is up to the process engineer to determine the best sensor installation location.
Cleaning & Calibration
During maintenance activities, be certain to employ appropriate personal protective equipment and safety procedures.
All pH sensors require cleaning. They should be cleaned with a non-ionic detergent and rinsed with clean water. A soft-bristle brush can help remove stubborn buildup. A weak or dilute acid also can help remove scale and buildup. Proper sensor cleaning is critical to insure rapid response time and measurement stability. Thorough cleaning must be accomplished before sensor calibration to make certain that process residue has no impact on the pH measurement while the sensor is in buffer solutions.
Assuming the pH sensor is properly cleaned, rinsed and healthy, a two-point calibration in pH 4 and pH 7 buffer solutions will yield the most consistent slope and zero point throughout a broad temperature range. Reference buffer tables to verify response to pH buffers above pH 9 as the actual pH of these buffers can change significantly with temperature.
Prior to calibration, new sensors or sensors that have been properly stored for extended periods should be soaked in pH 4 buffer for at least 30 minutes.
Maintenance intervals and activities depend on actual process conditions, sensor type, maintenance performed and handling. Slow response, low slope and significant zero shift all are indicative of the need for cleaning, calibration or sensor replacement. Ultimately, experience and performance requirements will dictate maintenance requirements and useful sensor life.
Download: Here